Light-emitting diode is a special diode. Like ordinary diodes, light-emitting diodes are composed of semiconductor chips. These semiconductor materials are pre-implanted or doped to produce p and n structures.
Like other diodes, the current in the light-emitting diode can easily flow from the p pole (anode) to the n pole (cathode), but not in the opposite direction. Two different carriers: holes and electrons flow from the electrodes to the p and n structures under different electrode voltages. When holes and electrons meet and recombine, the electrons fall to a lower energy level and release energy in the form of photons (photons are what we often call light).
The wavelength (color) of the light it emits is determined by the bandgap energy of the semiconductor materials that make up the p and n structures.
Since silicon and germanium are indirect bandgap materials, at room temperature, the recombination of electrons and holes in these materials is a non-radiative transition. Such transitions do not release photons, but convert energy into heat energy. Therefore, silicon and germanium diodes cannot emit light (they will emit light at very low specific temperatures, which must be detected at a special angle, and the brightness of the light is not obvious).
The materials used in light-emitting diodes are all direct bandgap materials, so the energy is released in the form of photons. These forbidden band energies correspond to the light energy in the near-infrared, visible, or near-ultraviolet bands.
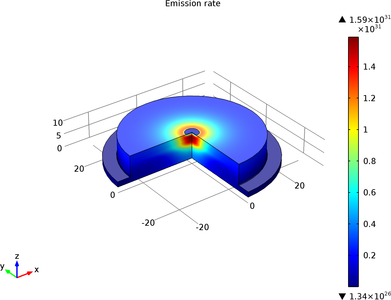
This model simulates an LED that emits light in the infrared part of the electromagnetic spectrum.
In the early stages of development, light-emitting diodes using gallium arsenide (GaAs) could only emit infrared or red light. With the advancement of materials science, newly developed light-emitting diodes can emit light waves with higher and higher frequencies. Today, light-emitting diodes of various colors can be made.
Diodes are usually constructed on an N-type substrate, with a layer of P-type semiconductor deposited on its surface and connected together with electrodes. P-type substrates are less common, but are also used. Many commercial light-emitting diodes, especially GaN/InGaN, also use sapphire substrates.
Most materials used to make LEDs have very high refractive indices. This means that most of the light waves are reflected back into the material at the interface with the air. Therefore, light wave extraction is an important topic for LEDs, and a lot of research and development is focused on this topic.
The main difference between LEDs (light emitting diodes) and ordinary diodes is their materials and structure, which leads to significant differences in their efficiency in converting electrical energy into light energy. Here are some key points to explain why LEDs can emit light and ordinary diodes cannot:
Different materials: LEDs use III-V semiconductor materials such as gallium arsenide (GaAs), gallium phosphide (GaP), gallium nitride (GaN), etc. These materials have a direct bandgap, allowing electrons to directly jump and release photons (light). Ordinary diodes usually use silicon or germanium, which have an indirect bandgap, and the electron jump mainly occurs in the form of heat energy release, rather than light.
Different structure: The structure of LEDs is designed to optimize light generation and emission. LEDs usually add specific dopants and layer structures at the p-n junction to promote the generation and release of photons. Ordinary diodes are designed to optimize the rectification function of current and do not focus on the generation of light.
Energy bandgap: The material of the LED has a large bandgap energy, which means that the energy released by the electrons during the transition is high enough to appear in the form of light. The material bandgap energy of ordinary diodes is small, and the electrons are mainly released in the form of heat when they transition.
Luminescence mechanism: When the p-n junction of the LED is under forward bias, electrons move from the n region to the p region, recombine with holes, and release energy in the form of photons to generate light. In ordinary diodes, the recombination of electrons and holes is mainly in the form of non-radiative recombination, that is, the energy is released in the form of heat.
These differences allow LEDs to emit light when working, while ordinary diodes cannot.
This article comes from the Internet and the copyright belongs to the original author
Post time: Aug-01-2024